Plasma nitriding theory (1):Basic knowledge of plasma
- Doctor X
- Oct 8
- 2 min read
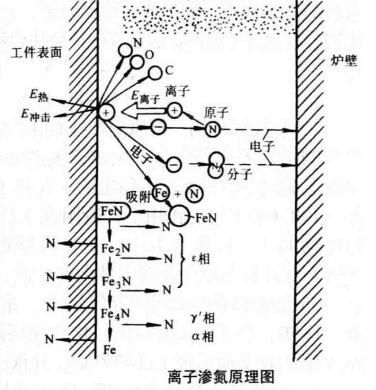
Plasma nitriding is carried out in a vacuum furnace by discharging rarefied gas to form a local ionized state composed of ions, electrons and neutral particles. Therefore, it is very necessary to understand the generation and discharge characteristics of plasma. Let's begin with the plasma nitriding theory.
I. Basic Concepts of Plasma
Plasma refers to an ionized gas, consisting of ions, electrons, and neutral particles. It appears neutral overall. It is an ionized state composed of charged particles and is known as the fourth state of matter.

II. Plasma Generation
To generate plasma, neutral particles must be ionized. There are various methods for ionizing neutral particles, three of which are listed below.
1. Low-pressure gas discharge to generate plasma. Ion nitriding furnaces utilize this method.
2. Methods utilizing high-energy particles, such as those used in nuclear fusion.
3. Methods utilizing electromagnetic wave energy, such as using X-rays or γ- rays to ionize gases.

III. Properties of Plasma
1. Plasma is electrically neutral on a macroscopic scale. It is an ionized gas composed of three components: electrons, ions, and neutral particles. The total charge of electrons and ions is essentially equal, making it point-neutral as a whole.
2. Plasma has a high electrical conductivity. Although plasma is electrically neutral on a macroscopic scale, it possesses excellent electrical conductivity due to the large number of charged particles (electrons and ions) contained in the system and their high concentration.
3. Plasma chemical reactions are easier to carry out than thermochemical reactions. Plasma chemical reactions are characterized by thermodynamically stable excited and ionic states. The activation energy for most such reactions is very low. For example, in ion-molecule reactions, the activation energy is usually zero, making the reaction easier to carry out and becoming diffusion-dominated.

Comments